Sex determination
Surprisingly, it is only in the last 50 years that we have begun to understand the nature of the biological events which determine our sex, (and for that matter, why we bother with sex at all and why two sexes are better than three or more). It is not so long ago that women were blamed if they failed to produce a son for their husband and clearly it was thought that the power of sex determination lay within the body of the woman. During this century the chromosomal basis of human sex determination has been demonstrated and in the last few years some of the genes responsible have been identified.
The sexual identity of an individual is determined at several levels, chromosomal sex, gonadal sex, somatic sex and sexual orientation.
sex chromosomes
The chromosomal basis of sex determination in humans was recognized when metaphase chromosomes from dividing male and female cells could be studied and counted. The normal karyotype contains 46 chromosomes including either two X chromosomes (46XX, females) or one X chromosome and one Y chromosome (46XY, males). Individuals with 45X or 47XXX karyotypes are female, individuals with 47XXY karyotype are male. Therefore it can be deduced that the Y chromosome is sex determining
sex determination
Experiments involving the removal of the embryonic gonad have revealed that in mammals, no matter what the chromosomal sex of the somatic cells, the body will develop as a female unless a male gonad is present to secrete mullerian inhibiting substance and testosterone. This can be partially mimicked in the genetic condition testicular feminisation in which the gene coding for the androgen receptor is not expressed so that, although the testis in an XY individual secretes testosterone, the somatic tissues are unable to respond to it. Consequently the individual's body develops as a woman but with internal testes instead of ovaries. In 1990, a Y encoded gene SRY was discovered which (at least in mice) is able to transform the sex of an XX embryo from female to male. Individuals with mutations in this gene develop as females despite having an XY chromosomal constitution. About one male in 10,000 does not appear to have anY chromosome but instead has two X chromosomes. These XX males can frequently be shown to have inherited from their fathers an X chromosme onto which a little bit of the Y chromosome carrying SRY has been transfered by an "illegitimate" cross over. XX males are entirely normal except that they are infertile and their heights are in the normal female range rather then the male.
In other organisms things happen differently. Both Drosophila and the nematode Caenorhabditis elegans use a mechanism in which each cell measures the relative number of X chromosomes compared to the number of autosomes. However, the genes involved in the counting process and in its interpretation do not seem to be related in the two species.
Even within vertebrates there are a variety of sex determination mechanisms.
- Birds use a chromosomal system, however unlike the mammalian system, males are homogametic ZZ and females are heterogametic ZW.
- Alligators are chromosomally the same in both sexes, they determine sex by the temperature at which embryos are allowed to develop. If warm then males are formed and if cool then females are formed.
sexual identity
It is a much debated question as to whether our own sense of sexual identity is genetic or environmental in origin. Like most complex phenotypes it probably can be either but is usually both! Recent controversy centred on the results obtained by Hamer who showed evidence for one such gene, a recessive gene on the X chromosome, which when mutant may make its bearer, if male, more likely to be homosexual.
Sex linkage
sex linked recessive genes
Genes carried on the X chromosome have a distinctive pattern of inheritance. Because males are hemizygous, i.e. they have only one copy of the X chromosome, and because the Y chromosome carries very few genes (though those which it carries are often homologous to X linked genes) then recessive mutations manifest themselves in the phenotype of males. If the mutant gene is lethal (such as Duchenne Muscular Dystrophy) then it takes an unusual event to produce an affected female.
An X linked pedigree

A typical pedigree will show clusters of affected males (each brother will have a 50% chance of being affected) connected through unaffected carrier females. There will be no cases of direct male to male transmission because males transmit their X chromosomes to their daughters and not to their sons.
The following passage is quoted from The history of haemophilia by Dr. P.L.F. Giangrande
The story of Queen Victoria
Haemophilia is sometimes referred to as the Royal disease. Queen Victoria had no ancestors with the condition but soon after the birth of her eighth child, Leopold, in 1853 it became evident that he had haemophilia. Queen Victoria was thus an example of how the condition can arise as a spontaneous mutation. Leopold's medical condition was reported in the British Medical Journal in 1868, and it is clear that he was troubled by bleeds occurring at least once a month. He died at the age of 31 in 1884 from intracerebral haemorrhage after a fall. Leopold had married two years before his death. His daughter, Alice, was an obligate carrier and also went on to have a haemophilic son. Rupert, Viscount Trematon, was born in 1907 and died at the age of 21, also from an intracerebal haemorrhage. It also subsequently transpired that two of Queen Victoria's own daughters, Alice and Beatrice, were carriers of haemophilia. The condition was transmitted through them to several Royal families in Europe, including Spain and Russia. Perhaps the most famous affected individual was the son of Tsar Nicholas II of Russia. The story of the young Tsarevich Alexis, who was born in 1904, has been the subject of a Hollywood film as well as a novel by Dorothy Sayers ("Have his carcase": 1932). There has been speculation that the illness led to severe strain within the Royal family, and enabled Rasputin to gain influence on the family. Alexis and his family were murdered by the Bolsheviks in 1917. The haemophilic gene has now died out in these Royal families, emphasising the severity of the condition in the absence of effective medical treatment. Thus we do not know to this day if the condition was haemophilia A or B.
mutation rate of X linked genes
One third of all X chromosomes are present in males and hence one third of mutant X chromosomes are present in males. Consequently, if the condition is lethal, then one third of the mutant X chromosomes will be lost from the population each generation. If the frequency of the disease is not changing then the lost mutant chromosomes will have to be replaced by new mutation. Consequently, the mutation rate of a lethal X linked recessive disease is one third of the frequency of the disease.
sex linked dominant genes
Sex linked dominant conditions are extremely rare, examples include incontinentia pigmenti (which is lethal in males) and congenital generalized hypertrichosis (wolf man syndrome).
X inactivation
All cases of abnormal karyotypes in which a single autosome is missing (autosomal monosomy) are lethal during embryogenesis even for
the smallest autosomes. Yet males with only one X chromosome (a medium sized
chromosome) are (comparatively!) normal. How is this accomplished? The answer
(as suggested by Mary Lyon in 1961) is by inactivation of one of the two X
chromosomes in females so that the normal state for a cell is to have two active
sets of autosomes and only one active X chromosome. The other X chromosome is
condensed and inactive and is visible as a dark staining "Barr body" pushed
against the nuclear membrane. The following image of cells from a female cheek
swab was "borrowed" from the US
Army.
Three nuclei can be seen each with a characteristic dark blob (which is particularly clear in the centrally located nucleus).
Mary Lyon hypothesised that the X inactivation happened at random early in development so that each female is composed of two populations of cells. In one population one X chromosome is expressed and in the other, the second X chromosome is expressed. Females are thus mosaics, i.e. composed of two genetically distinct cell populations. For genes which are homozygous this will make no difference but for genes for which the female is heterozygous the two populations of cells will be of opposite phenotypes. In this way she explained the patterns of hair colouration in, for instance, the tortoiseshell cat which is always female except for the very rare occurence of an XXY male, which exception proves the rule!
Sex limited traits
As we have seen, sex linked traits are generally expressed much more often in males than in females. You should be aware however that some traits which affect one sex more than another are not necessarily sex linked. Examples are cases of sex limited expression which might include genes affecting beard growth or breast size, and (in cattle), horn growth and milk yield. These genes have no visible affect in one sex because the necessary machinery to express them is not present.
Sex influenced traits
On the same general line as sex limited traits are sex influenced traits. Pattern baldness is a condition which is dominant in men but recessive in women.
Imprinting
Some genes are inactivated when transmitted through one sex. Angelman syndrome and Prader-Willi syndrome are two different conditions both of which seem to be caused by very similar deletions of a small part of chromosome 15.
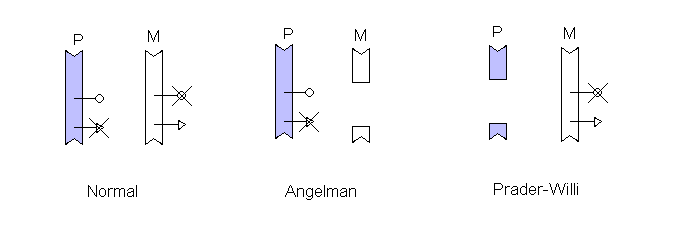
In this diagram, two genes are shown in the critical region. Each is inactivated by imprinting, the Angelman syndrome gene is turned off on the chromosome inherited from the father while the Prader-Willi gene is turned off on the maternally transmitted chromosome. When a deletion covering the region is inherited on the other chromosome one syndrome or the other results.
Page initially found at: Chemistry of the cell and genetics, Lecture 4