The Molecules of Life
Proteins
Proteins are the primary building materials of
the body. Your hair, skin, muscles, and organs are composed mostly of
proteins. Proteins are strong yet flexible, and they have a complex
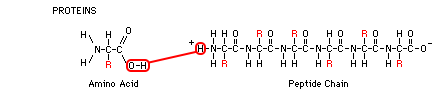
3-D structure. Amino acids are the basic building blocks of proteins. Amino acids have an NH2 (amine) group on one end, a H-O-C=O
(carboxyl) group on the other end, making it acidic, and an R group
which extends from the central carbon atom. The chemical makeup of this R group varies from one amino acid to another and gives each amino acid its unique properties. There are 20 amino acids that
are important to humans, and all proteins are made from combinations
of these subunits. Chains of amino acids are called peptides. In the poly-peptide chain shown below, can you see the individual amino acids that are strung together in a repeating N-C-C pattern? Between the terminal C of one amino acid and the N of the next one, energy from ATP is used to pull the O-H from the C, and the H from the N, forming H2O and joining them in a peptide bond, lengthening the chain. When we get to the genetics section of the course, we will study protein synthesis. That's the process by which DNA instructions are transcribed into RNA, which is then translated into the amino acids that are strung together to form long poly-peptide chains. These chains are then woven together like strands in a rope or like threads in a blanket to form various proteins. When food is consumed, the proteins are broken down into their constituent amino acids and rebuilt into the proteins of the body. However, excess amino acids are not stored for future use, and the body only starts to break down its own proteins during starvation, when the ordinary sources of fuel (fats and carbohydrates) are not available.
Dehydration Synthesis and Hydrolysis: Proteins, fats, and carbohydrates all use these two common reactions involving water to assemble and disassemble the molecule. When two hydrogens and one oxygen are removed from two separate molecules and the result is a single molecule and a water, this is called a dehydration synthesis reaction. The molecules are "dehydrated" because water is removed, and they are synthesized (joined) into one large molecule. When one large molecule is split (lysis means splitting) into two molecules with the addition of water and energy, the reaction is called hydrolysis. In each of the illustrations, these reactions, which are opposites, are shown in red.
Fats (lipids)
Fats are the primary long-term energy storage molecules of
the body. Fats are very compact and light weight, so they are an
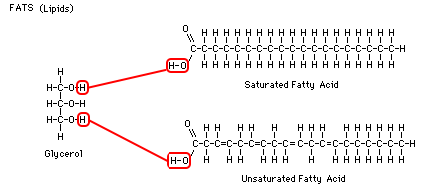
efficient way to store excess energy. A fat is made up of a glycerol,
which is attached to 1 to 3 fatty acid chains. Most of the energy from fats comes from the many carbon bonds in these long, fatty acid chains. Fatty acids connect to glycerol in the region where each molecule has an -O-H group. Two hydrogens and one oxygen are split off, forming H-O-H (water) and the long carbon chain is attached to the glycerol. Each glycerol can carry up to three fatty acid chains, which would make it a "tri-glyceride." When each fatty acid is attached to glycerol, a water molecule is produced. To reverse the reaction and split the fatty acid from the glycerol, just add water and energy.
Heart health and fats: Saturated fatty acids have
no double bonds and therefore hold the maximum number of hydrogens. In other words, the carbons are "saturated" with hydrogen. Unsaturated
fatty acids have some double bonds and therefore hold fewer
hydrogens. Saturated fats are not as good for you as unsaturated
fats. Saturated fats are long straight molecules that can clog your arteries, whereas more unsaturated fats, because of the additional double bonds, are more bendy and less likely to clog up the small blood vessels. It's like the difference between trying to swallow an uncooked spaghetti stick and a cooked spaghetti noodle.
Carbohydrates
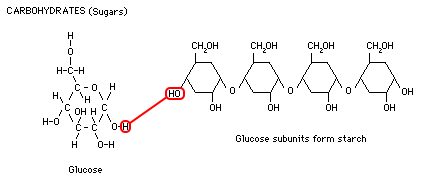
Glucose, a 6-carbon sugar, is a simple carbohydrate or "mono-saccharide." Sugar is a source of quick energy for the body because it is easily metabolized (broken down). Larger, more "complex carbohydrates" are made by stringing together chains of glucose subunits into di-saccharides, tri-saccharides, poly-saccharides. Starch is a complex carbohydrate which plants create for energy storage, and is the most common carbohydrate in the human diet. Foods like potatoes, corn, rice, and wheat are rich in starch. Animals break the starches back down into glucose subunits and convert the glucose into glycogen for storage. Glycogen is a complex storage molecule made from glucose using insulin. Diabetics, who lack insulin, cannot make glycogen so they excrete excess sugar in their urine. Glucose is broken down through a process called glycolysis (lysis means splitting) in order to release energy stored in the carbon-carbon bonds.
Nucleic Acids
These molecules contain the genetic code,
which has all the information necessary to build the body. The basic
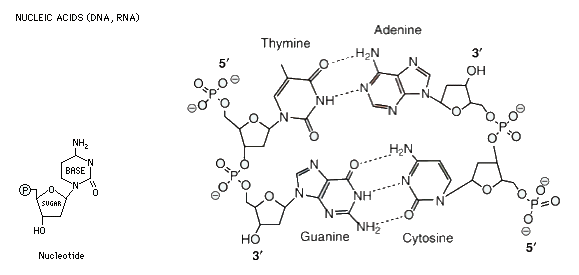
unit is called a nucleotide, which is composed of a sugar-phosphate
backbone attached to one of four nitrogenous bases; cytosine, guanine, adenine or thymine. C joins to G, and G to C by three hydrogen bonds, indicated by the dotted lines. A joins to T and T to A by two hydrogen bonds. Note that the DNA molecule shown below is double stranded, and that the two strands run in opposite directions, denoted by the 3' and 5' ends. While nucleic acids are important as information carrying molecules, they are not nutritionally important.