Chapter 5
Metabolism -- The sum of all chemical reaction within a cell. It can also be described as catabolism + anabolism.
Chemical Reactions
Some reactions require energy. Energy must be added in order to make
these reactions happen and the product(s) will be at a higher energy level
than the reactants. In metabolism, many anabolic reactions fall
into this category. Anabolic reactions require energy. Catabolic
reactions release energy.
Not all energetically favored reactions are spontaneous. Many times some energy of activation needs to be added. For example, paper (cellulose = C6H12O6) exists stably in the presence of oxygen. Even though the rapid oxidation of the cellulose to form CO2, H2O and C is energetically favored, the paper won't burn (burning = the rapid oxidation of cellulose) unless activation energy (heat) is applied.
I. ENZYMES
In the cell, the energy needed to drive anabolic reactions as
well as the activation energy needed to get many catabolic reactions
going cannot be directly applied as heat. Instead, cells use enzymes
to lower the amount of energy needed to cause the reactions to occur. Thus
enzymes
are called catalysts because the facilitate reactions and speed
them up but they don't enter into the reactions.
Enzymes lower the activation energy of reactions because enzymes are able to (1) bind to the reactants (substrate), (2) force the reactants (substrate molecules) very close to each other and (3) bend the substrate molecules and destabilize their electron configurations. This makes the molecules unstable and reactive.
II. Components of an Enzyme:
- The place on the enzyme where substrate binds is called the substrate binding site or the active site of the enzyme. Allosteric site is a site other than the active site.
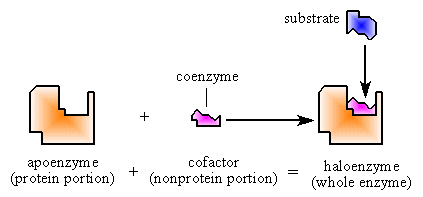
- Apoenzyme = the protein portion
- Cofactors = are non-protein atoms or molecules which bind to the apoenzyme. They are divided into organic molecules = coenzymes, and inorganic elements = metal ions.
- Coenzymes= NAD+ (nicotinamide adenine dinucleotide), FAD (flavin adenine dinucleotide), CoA (coenzyme A)
- Metal ions = Iron, copper, calcium, zinc, magnesium.
-
Holoenzyme = Apoenzyme + Cofactor
III. Factor affecting enzyme function:
(don't forget saturation!)
1) pH
2) Temperature
3) Substrate concentration
4) Enzyme concentration
IV. Enzyme Inhibition:
a) Competitive Inhibition: A molecule with similar structure
to the normal substrate can occupy (and block) the enzyme's active site.
Can be reversed by adding more substrate. E.g. folic acid synthetase binds
PABA ---> folic acid. The drug sulfanilamide has a chemical structure very
similar to PABA and the drug will bind to the active site of the enzyme.
Folic acid synthetase however is incapable of converting sulfanilamide
into anything.
b) Non-competive Inhibition: Inhibitors (e.g. lead or other metals) can bind to the allosteric site changing the shape of the enzyme. Now, the active site is different and can't bind to the substrate.
ENERGY FLOW IN METABOLISM
In most of the oxidations and reductions which we will study, electrons
(e-) will be moved with protons (H+). Watching the hydrogens therefore
provides a convenient way to tell if a molecule has been oxidized or reduced.
Also, in many of the oxidation-reduction reactions we will look at,
the molecule nicotinamide adenine dinucleotide (NAD) which serves
as an electron-shuttle. NAD can become REDUCED to NADH2,
and then carry the electrons to some other reaction and become OXIDIZED
back to NAD. In other words, NAD can pick up electrons from
one reaction and carry them to another.
Note that when a molecule gets OXIDIZED IT LOSES ENERGY. Also,
the more reduced a molecule is the more energy it contains. (See pgs 121-122,
figs. 5.8, and 5.9 for descriptions of NAD and oxidation-reduction reactions.)
The ultimate goal in many instances of catabolism will be to take energy
from a (food source) molecule, trap the energy and store it as ATP.
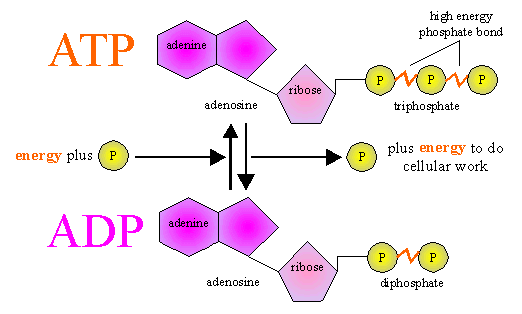
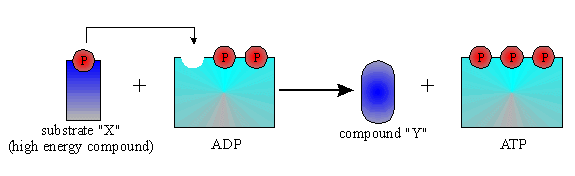
There are three ways to make ATP:
1.) Substrate level phosphorylation- where a high energy phosphate
from an intermediate phosphorylated metabolic molecule gets
transferred directly onto
ADP in a catabolic pathway converting
it to ATP.
3.) Photophosphorylation - This is seen only in cells carrying
out photosynthesis. In here, light energy is used to generate electrons
and then the energy is extracted from the electrons by an electron transport
chain. As in oxidative phosphorylation, the extracted energy is used
to make ATP by chemiosmosis.
BACTERIAL METHODS OF OBTAINING ENERGY
The respiration of glucose as a fuel source occurs in 3 stages: glycolysis,
Krebs cycle and electron transport chain.
Glucose + 6O2 ----> 6CO2
+ 6H2O + energy
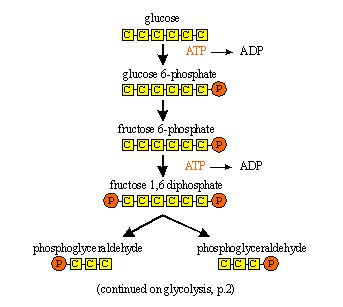
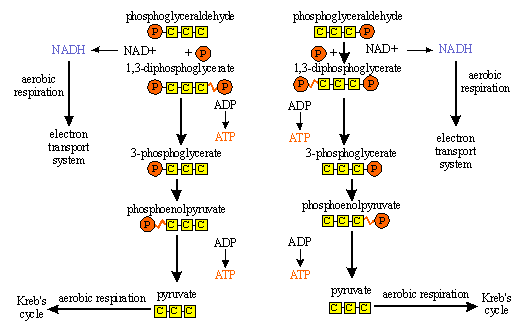
(a) GLYCOLYSIS - or Embden Meyerhof Pathway
The electron transport chain enzymes are a series of oxidation-reduction
electron carrier molecules and proton pumps. These enzymes use the energy
in the electrons from glycolysis and Krebs cycle to move protons against
a concentration gradient to form the proton motive force.
In the mitochondria of eukaryotes, 3 pairs of protons are "pumped
out" between the inner and outer mitochondrial membranes during a single
run down the electron transport system and their re-entry generates the
formation of 3 molecules of ATP. However, in prokaryotes, often
less protons are transported across the membrane in a single run (2 pairs
in E. coli) so less ATP's are generated (2 in E. coli). The
principle is however, the same.
(2) FERMENTATION:
A lot of ATP is produced from NADH2 and FADH2 Comparison between Fermentation and Aerobic respiration.
Summary of Aerobic respiration:
Summary of Metabolism:
CLASSIFICATION OF ORGANISMS BY NUTRITIONAL PATTERN:
Energy is the ability to do work. Bacteria require energy for
motility, active transport of nutrients into the cell, and biosynthesis
of cell components such as nucleotides, RNA, DNA, proteins, peptidoglycan,
etc. In other words, energy is required to drive various chemical reactions.
To get energy, bacteria (chemoheterotrophs) take energy-rich
compounds such as glucose into the cell and enzymatically break them down
to release their energy. Therefore, the bacterium needs a way to trap
that
released energy so it is not wasted as heat and store the energy in a form
that can be utilized by cells. Principally, energy is trapped and stored
in the form of adenosine triphosphate or ATP. Much ATP is
needed for normal growth. For example, a typical growing E. coli cell must
synthesize approximately 2.5 million molecules of ATP per second to
support its energy needs.
1) ENERGY SOURCE
Chemoheterotrophs= energy and carbon from organic molecules
Energy in metabolism often flows in terms of electrons. If electrons
ARE LOST, this is called oxidation. If electrons ARE GAINED, this
is called reduction. Oxidation is coupled to reduction; that
is, if something gets oxidized, then something else gets reduced (remember
the first and second laws of thermodinamics!).
2.) Oxidative phosphorylation - where a (food source) molecule
is oxidized and the energy is extracted from the electrons by an electron
transport chain.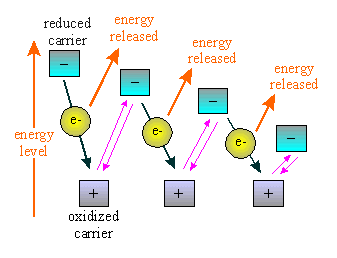
The extracted energy is then used to make ATP by a process known
as chemiosmosis.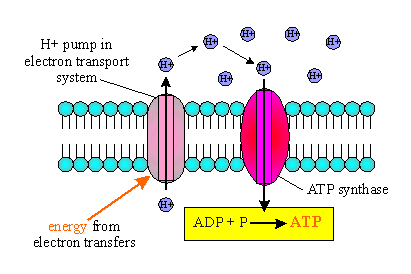
1. AEROBIC RESPIRATION
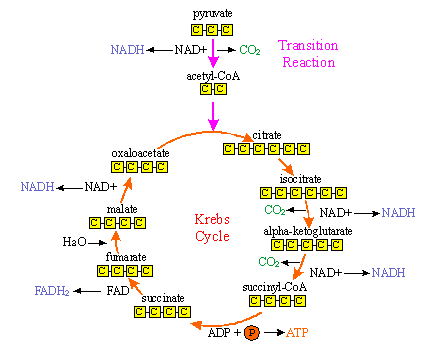
(b) KREBS CYCLE
(c) ELECTRON TRANSPORT CHAIN
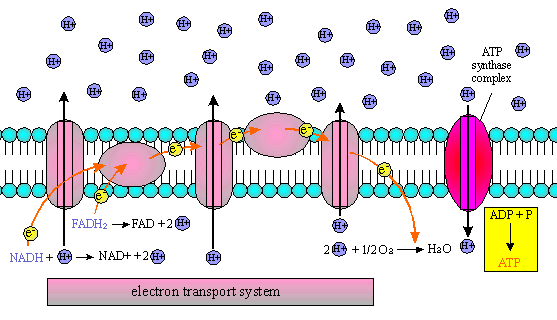
It is a series of enzymes imbedded in a membrane. These enzymes use
the membrane to set up a chemiosmotic gradient of hydrogen ions. This gradient
of hydrogen ions is called a proton motive force and this force
supplies the energy for an ATP synthetase.
(3) RESPIRATION
Glycolysis
Krebs Cycle
Electron Transport
Total net output
ATP produced
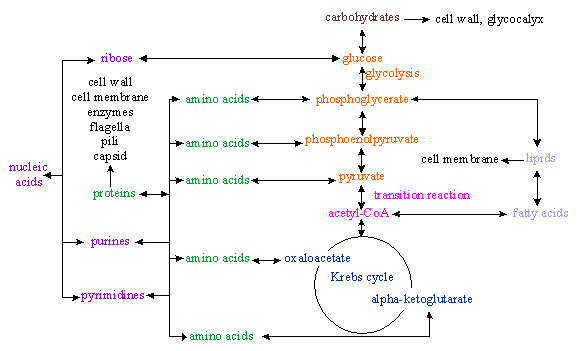
2) CARBON SOURCE
EXAMPLES:
Chemoautotrophs= energy from reduced inorganic compounds and
CO2 as source of carbon.