CHAPTER 6 - Microbial Growth
Note: These notes are outlines of my lectures and they in no way represent all the material covered in class!!Physical requirements for the growth of microorganisms
WHAT FACTORS AFFECT GROWTH?
- Physical factors - temp, pH, water, oxygen, pressure
- Nutrients - Cell constituents and energy sources
1. Temperature:
How does temperature affect optimal growth?
- minimum, optimum, maximum temperatures for growth
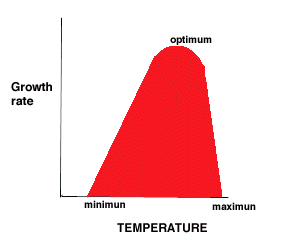
- Minimum - no growth below this temperature
- enzymes don't function
- transport ceases
- cells dormant not dead
- dangers of freezing and thawing
- Optimum - closer to maximum than minimum (5 to 10 C below max)
- Maximum - Bacterium A grows at temperatures up to 40°C. What might be happening to the cell at 41°C?
- Categories based on optimum temperatures:
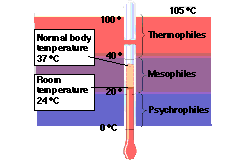
- psychrophiles <20 C
- high levels of unsaturated fatty acids --> remain fluid at low temperature
- mesophile 20-40 C
- thermophile >40 C and < 65 C
- extreme thermophiles > 65 C
- hot water heaters, hot springs, vents
- E.g. Pyrodictum, min 82 opt 105 max 110 C
- extremes have lipids, proteins adaptation
- PCR - Thermus aquaticus
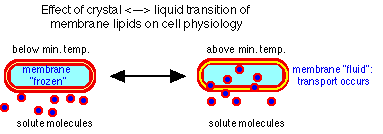
- require hydrogen ions for membrane integrity
- do not survive at neutrality
- prevent uptake into cytoplasm and pump out
- pump in protons
- generate ammonia (+) by ammino acid degradation
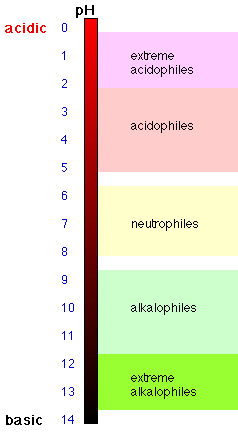
| Bacteria | min pH | opt pH | max pH | |
| Thiobacillus | 1.0 | 2-2.8 | 4-6 | |
| E. coli | 4.4 | 6-7 | 9.0 | |
| C. sporogenes | 5.4 | 6-7.6 | 9.0 | |
| P. aeruginosa | 5.6 | 6.6-7 | 8.0 | |
| Nitrobacter | 6.6 | 6.6-8.6 | 10.0 | |
3. Classification based on water activity/osmotic pressure (remember, low water activity = high osmotic pressure)
- halophiles- require sodium >9%; extremes may live in 30%
- marine salt about 3%
- cell wall and membrane fall apart w/o sodium
- nonhalophile - 0 to 1.5%
How do microbes adapt to low water activity?
- Microbes can change their internal osmotic environment
- E. coli in GI tract
- produce or import compounds that increase internal solutes
- compatible solutes - do not harm host
- ions, sugars, amino acids
4. Oxygen:
How does oxygen affect optimal growth?
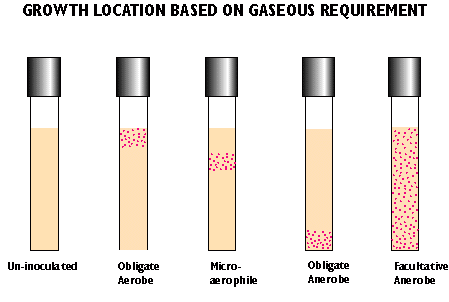
- aerobes - require oxygen
- obligate/strict anaerobes - killed by oxygen
- aerotolerant anaerobes - does not require oxygen but not killed
- facultative anaerobe - grows in presence or absence of oxygen with different metabolic strategies
- microaerophiles - require reduced levels of oxygen
Why is oxygen toxic to some microbes?
- metabolic byproducts all organisms produce are toxic to cells
- hydrogen peroxide
- superoxide
- present in WBC to kill bacteria
- aerobes have catalase and/or peroxidase and superoxide dismutase (SOD)
- catalase: 2H202 -> 2H20 + 02
- peroxidase: H202 + 2H+ -> 2H20
- SOD: 202- + 2H+ -> 02 + H202
- strict anaerobes lack these enzymes
- microaerophiles either reduced amounts of these enzymes or oxygen-sensitive forms of enzymes
- aerotolerant have these enzymes but not those used in aerobic metabolism
- Toxic forms of oxygen are broken down by several enzymes; one of these is catalase. This enzyme breaks down hydrogen peroxide to form water and molecular oxygen. Our tissues as well as many microorganisms are catalase positive - thats why the bubbles when you pour peroxide on a cut.
Why would our tissues have peroxide in them?
Some theories of cancer hypothesize that oxidants (like toxic forms of oxygen) damage DNA and thus cause the mutations which in turn cause cancer. These theories predict that antioxidants such as beta-carotene, vitamin E and vitamin C would thus have anti-mutation activity.
commonly called vitamins, these are organic substances required for the growth of an organism but which the organism can not synthesize.
What is the difference between a defined and an undefined medium?
WHAT FACTORS AFFECT GROWTH?
- Nutrients - Cell constituents and energy sources
- Physical factors - temp, pH, water, oxygen, pressure
I. Nutrients:
| ELEMENT | CELL FUNCTION | ||||||
| C | backbone of organic cell components, energy | ||||||
| H | water, organic components, pH, hydrogen bonds, re-dox | ||||||
| O | water, organic components, respiration | ||||||
| N | amino acids, nucleotides, coenzymes, ATP | ||||||
| S | amino acids, coenzymes, enzymes | ||||||
| P | nucleic acids, phospholipids, coenzymes, ATP | ||||||
| Fe | cytochromes, enzymes | ||||||
| Na, K, Ca, Cl, Mg, Mn | Trace elements: transport, ionic balance, cofactors (e- donor/acceptors) | ||||||
II. CLASSIFICATION OF ORGANISMS BASED ON carbon, energy, and electron sources
- Chemotrophs - Derives energy from chemicals
- Phototrophs - Derives energy from sunlight
- Autotrophs - Use carbon dioxide for carbon
- Heterotrophs - Use organic substrates for carbon
- Lithotrophs - Inorganic compounds for electrons
Bacterial Growth
- Bacteria grow by binary fission. Starting with one organism how many organisms would you have after 1,2,3,4,5,6,7,8,9,10,11,12 generations.
- Increase in cell numbers
- Binary fission
- Budding
- Fragmentation
- DNA duplication
- DNA repication
- single origin of replication, bidirectional
- theta intermediate
- Separation of DNA and cytoplasmic contents
- Cross wall formation
- time required for a cell to divide or a population to double
- 1 to 2 or 100 to 200 or 1 million to 2 million
- Most common bacteria have a generation time 30-60 min under opt. conditions.
- Most common pathogens in the body, about 5-10 hours.
- Why doesn't it grow immediately?
- What determines how fast it grows?
- Why does it stop growing?
- Lag phase - synthesis of new components or repair
- Log phase - reproduction at maximum rate (shortest generation time)
- exponential growth 1->2->4->8->16->32->64
- Stationary phase - no net increase, balance between cell division, cell "death", maintenance
- Death phase - do they really die?
If you started with 10 organisms, how many would you have after each of the above generations?
What is microbial growth?
| Mycobacterium tuberculosis (in lab) | 12 hrs |
| Clostridium botulinum (in lab) | 0.58 |
| E. coli (in lab) | 0.30 |
| E. coli (in mouse) | 20 hrs |
What happens when you inoculate a single bacterium from slant stored in refrigerator into nutritional medium and incubate?
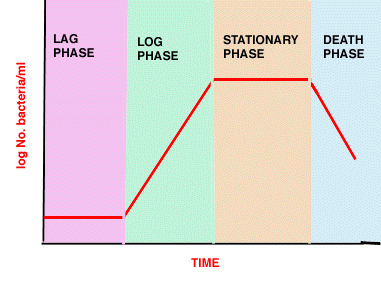
Phases in bacterial growth in batch culture
(see above picture)
Mathematics of growth- generation time
Growth equation:
n = log10Nf
- log10 Ni
0.301
Where n = number of generations, Nf = final
conc. of cell (e.g. 109/ml),
Ni = initial conc. of cells (e.g. 103/ml), and
0.301
is factor to convert log2 to log10.
cells/ml. Calculate n = (5 - 4)/0.3 = 1/0.3 = 3.33 generations.
Generation time: Total time = 6 hours = 360 minutes/3.33
generations = 108 minutes/generation
Conclude: generation time = 108 minutes
Note: be able to calculate g.t. Pay attention to units!
Graphical measurement of growth
· Plotting # of cells vs. time gives
a curved line.
· Plotting log # of cells vs. time
gives a straight line --- easier to interpolate, use.
· Plotting # of cells vs time on
semilog paper also gives a straight line --- easiest way in
practice to work with growth measurements.
· Note: often what is plotted on
the Y-axis of semilog paper is not # of cells, but something
more easily measurable, such as Absorbance (see
below).
.
CULTURE MEDIA
A culture medium is any material prepared for growth of an organism
in a laboratory setting. Microbes that can be cultured on a petri-plate
or in a test-tube containing media are said to grow under in
vitro conditions ("within-glass".)
It was not until the era of Robert Koch and his coworkers that Agar was introduced as a a common medium for bacterial growth. Agar is a complex polysaccharide derived from a marine sea weed. Few bacteria possess enzymes capable of digesting agar and therefore it is useful as a solidifying agent and for isolating microbes in pure culture. Prior to the advent of agar, gelatin was used as a growth medium. Unfortunately, many bacteria possess enzymes that liquify gelatin and therefore this medium is not useful for isolating pure cultures. However, gelatin liquefaction is one among a series of biochemical tests that helps differentiate species of bacteria.
What is a PURE CULTURE?
- A pure culture represents a single species (clonal in nature) of microorganisms
- A clone is a genetically identical population of microbes that have descended from a single parent cell
- Colonies are visible clones that have grown on solid media and represent millions of bacterial cells
- Distinctive characteristics of colonies should be noted such as:
- pigmentation
- odor
- elevation
- margin (border of the colony)
- consistency, such as mucoid, irridescence, filamentous, etc.
a) Chemically defined media: exact chemical composition is known.
Such media is often
commercially prepared.
b) Selective media. Contain chemicals which encourage growth
of certain types of microbes
but inhibits the growth of others.
c) Differential media allows different microbes to be
distinguished on the basis of various
biochemical reactions. Fermentation reactions involving
the catabolism of various sugars are
particularly useful biochemical tests
- Note: Many media are both selective and differential, such as MacConkey
(Mac) agar and Mannitol Salt agar (MSA).
d) Enrichment media contains a rich supply of nutrients
to encourage the encourage
growth of microorganisms. A commonly used enrichment
medium is blood agar. This
medium is also differential and it permits detection
of differnt patterns of hemolysis.
Measurement of growth
a) Total Cell count
· Petroff-Hausser chamber slide
--
needs large conc. (107 cells/ml minimum).
· Coulter Counter (for larger
microbes; fungi, yeasts, protozoa, etc.) --- uses electrical charge
difference in passing through small hole. Not so
useful with bacteria, get errors due to
clumping, debris, unable to differentiate bewteen
live and dead cells, etc.
b) Viable count
CFU (colony forming units) assay
1. carry out dilution series
2. plate known volumes on plates
3. count only plates with 30 - 300 colonies (best statistical accuracy)
4. extrapolate to undiluted cell conc.
Measures colony forming units (CFU), may or may not be same as number of cells --- accurate, but requires time for incubation.
Two ways to carry out viable count:
1. Spread plate: bacteria are spread on the
surface of agar using some sterile spreading
device. Advantages: if properly
carried out, all colonies should be easily counted.
Disadvantages: takes some
time, not always reliable in inexperienced hands, cells with low
tolerance to oxygen will
not grow. If "spreaders" are present may overgrow plate surface.
2. Pour plate: bacteria are mixed with melted
agar and cooled; colonies grow throughout the
agar. Advantages: almost
fail-proof technique, colonies well separated. Can allow growth
of organisms with lower
oxygen tolerance in agar. Disadvantages: colonies variable size,
harder to see similarity
in colony morphology between those on surface and in agar.
Counting may be more difficult.
Heat may kill some cells before agar cools and gels.
c) Light techniques
Often, can estimate cell numbers accurately by measuring visible turbidity.
Light scattered is proportional to number of cells. This only works above
cell densities of 107 in pure cultures. Eyeball method. This
is not a precise measurement, but shoud allow estimation within an order
of magnitude.
• no turbidity means less than 107 cells/ml
• Slight turbidity = 107 - 108 cells/ml
• high turbidity= 108 - 109 cells/ml
• Very. high turbidity = greater than 109 cells/ml (cultures
rarely get as high as 1010 cells/ml)
Absorbance (usually at wavelengths around 400-600 mn). Accurate
measure of cells when concentration not too high. Easy and quick to measure
(can sample in less than a minute).
d) Batch vs. Continuous culture methods
· Batch method: put small
inoculum of pure culture into sterile medium, let grow. Common lab
procedure, but not typical of many real environments.
• Continuous culture (see text section 6.11): use chemostat
or turbidostat.
Trickle fresh
medium into culture at slow but steady rate, displace
= volume of culture as overflow.
• Cells remain in exponential (but suboptimal) state, growing at known
rate. Good simulation for
study of many natural environments.