 Fernando P. Monroy
Fernando P. Monroy Fernando P. Monroy
Fernando P. MonroyImmunoparasitology: immunobiology of
host-parasite
relationships
Ph.D., University of Queensland, Australia
Assistant Professor - Indiana State University (1993-1999)
Associate Professor - Indiana State University (1999-2000)
Joined the Department in 2000
Research conducted by Dr. Monroy’s is directed towards clarification of the mechanisms employed by parasites to survive and establish successful infections. More specifically, I am interested in a group of parasitic organisms, the protozoa which includes the agents of such terrible diseases as malaria, toxoplasmosis and Chagas disease.
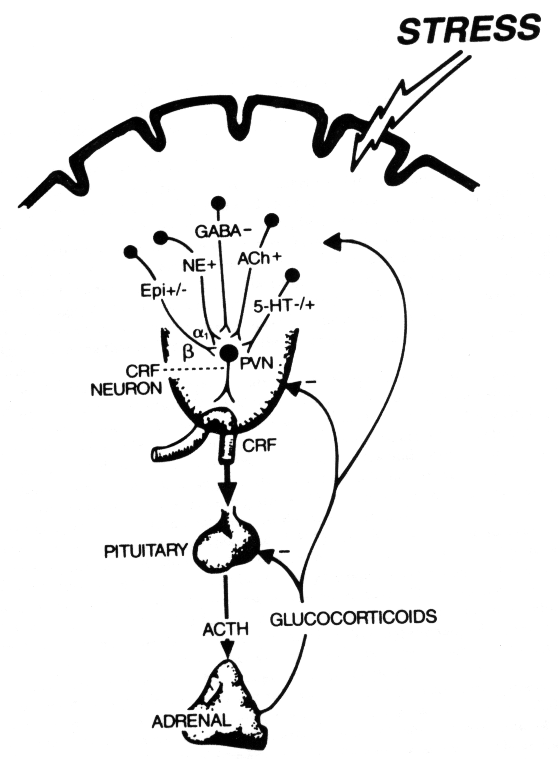
My first research interest involves the role of physiological stress in the pathogenesis of the obligated intracellular parasite Toxoplasma gondii. Stress in its wider interpretation, is a process central to the relationship between behavior and health. The physiological responses to stress are mediated through neural and endocrine systems. The two most likely routes by which the nervous system may communicate with cells of the immune system is by release of catecholamines from the sympathetic nervous system, and by secretion of glucocorticoid hormones following stimulation of the hypothalamic-pituitary-adrenal axis. My research goal intends to study the interactions between the nervous, endocrine and immune systems by using an infectious agent as a marker. The obligated intracellular parasite T. gondii is been used for two reasons: first, to investigate the effects of stress as a cofactor in the pathogenesis of an opportunistic infection in which cell-mediated immunity is of critical importance in host defense. Second, T. gondii is the most common opportunistic infectious agent of the central nervous system in HIV patients. Our interests center on the effects of stress in the modulation of regulatory cytokines [interferon (INF)-g , tumor necrosis factor (TNF)-a , interleukin (IL)-12 and IL-10] and neurohormones (glucocorticoids and cathecholamines) in both the acute and chronic phases of T. gondii infection.
Our second major area of research focuses on the neuro-immuno regulation of intestinal responses during T. gondii infection. Our long-range goal is to understand how stress hormones and neuropeptides regulate infection by the opportunistic parasite T. gondii. We will investigate the role of the nor-epinephrine (N-EPI), the main mediator of the sympathetic-adreno-medullary (SAM) system as cofactor in the intestinal pathology of mice orally infected under conditions of stress. The rationale behind this research centers in the fact that susceptible C57BI/6 mice died after peroral infection with T. gondii due to intestinal pathology driven in part by interferon (IFN)-y released by lamina propia (LP) CD4+ T cells; while cold water stress (CWS) enhanced the survival of these mice likely by decreasing CD4+ T cell-driven intestinal pathology. Our hypothesis center on adreno-sympathetic regulation of intestinal T cells activity in stressed animals, leading to altered intestinal immune responses during T. gondii infection. Areas of research center around: (1) determination ex vivo of the contribution of N-EPI, the main mediators of the sympathetic nervous system (SNS) on LP dendritic cells and CD4+ T cells during CWS; and (2) determine the contribution of N-EPI and peripheral sympathetic innervations on LP cellular responses during CWS and infection. Our research expects to determine the contributions of N-EPI to the stress-induced changes in intestinal cellular responses during peroral T. gondii infection. In addition to having basic application in understanding normal physiologic and host defensive processes modulated by the central nervous system, our research will be of great value in designing new therapeutic strategies aimed at curbing pathology induced by enhanced inflammatory responses.
Our research
is supported by NIH funding:
1) R21 AI060401, "Innate Intestinal Responses in Murine Toxoplasmosis"
2) R21 AI065350, "Toxoplasma gondii: Neuro-Intestinal Interactions"
NEURO-ADRENO-IMMUNE
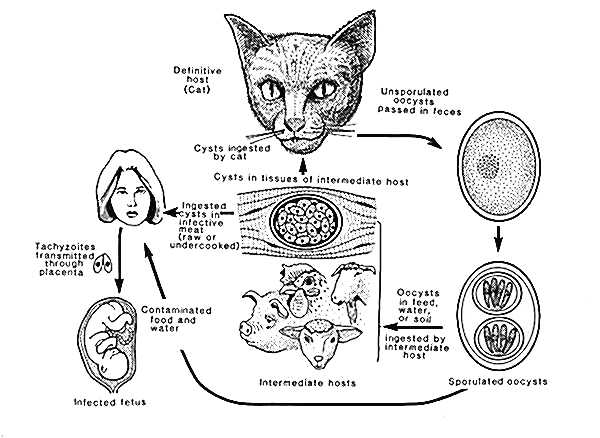
LIFE CYCLE OF
INTERACTIONS
Toxoplasma gondii
 |
 |
Selected
Publications:
MONROY, G.F. & ENRIQUEZ, F.J. (1992). Nematospiroides
dubius as a model for chronic intestinal parasitism.
Review Article for Parasitology
Today, 8(2): 14-17.
MONROY, G.F. & LOKER, E.S. (1993).
Production of
heterogenous carbohydrate-binding proteins by the host snail
Biomphalaria glabrata
following
exposure to Echinostoma paraensei and Schistosoma
mansoni.Journal
of
Parasitology, 79:
416-421.
MONROY, G.F. & DRESDEN, M.H. (1996).
Developmental
expression and role of cysteine proteinases in
Schistosoma
mansoni.International
Journal for Parasitology, 25: 109-114.
MONROY, F.P. & DUSANIC, D.G. (1997).
Mechanisms of
survival of Trypanosoma musculi in the kidneys of
chronically infected mice:
kidney
form reproduction and immunological reactions. Journal of
Parasitology,
83:848-852.
MONROY, F.P., MINNING, T. & DUSANIC, D.G.
(1998).
Trypanosoma
musculi survival in the kidneys of
chronically infected mice:
kidney
form ultrastructure , surface characteristics and serological
interactions.
Journal
of Parasitology,
84:
914-919.
BANERJEE, S., AVILES, H., FOX, M.T. &
MONROY, F.
(1999). Cold stress-induced modulation of cell immunity
during acute Toxoplasma
gondii
infection in mice. Journal of Parasitology, 85: 123-132.
AVILES, H., BELLI, A., ARMIJOS, R., HARRIS, E.
&
MONROY, F.P. (1999). PCR detection and identification of
Leishmania parasites
in clinical
specimens in Ecuador: a comparison with classical diagnostic methods. Journal
of Parasitology,
85:
147-153.
MONROY, F., BANERJEE, S., DUONG, P. &
AVILES, H.
(1999). Cold stress-induced modulation of inflammatory
responses and intra
cerebral
cytokine mRNA expression in acute murine toxoplasmosis. Journal of
Parasitology,
85: 878-896..
MONROY, F.P. & DUSANIC, D.G. (2000). The
role of
sequestered parasites in chronic infections. Review article for
Parasitology Today,
16: 107-110.
AVILES, H. & MONROY, F. (2001).
Immunomodulatory
effects of cold stress on mice infected intraperitoneally with
a lethal dose 50 (LD50) of Toxoplasma
gondii. NeuroImmunomodulation, 9: 6-13.
MORDUE, D.G., MONROY, F.P., LA REGINA, M.,
SCHREIBER,
R., DINARELLO, C. & SIBLEY, D. (2001). Acute
toxoplasmosis leads to
overproduction
of Th1 cytokines. Journal of Immunology, 167:4574-4584.
AVILES, H. & MONROY, F. (2001). Toxoplasma gondii: cold
stress-induced modulation of antibody responses.
Experimental Parasitology. 99:89-96.
AVILES, H., JOHNSON, M.T. & MONROY, F. (2004). Effects of cold
stress on spleen cell proliferation and cytokine
production during chronic
Toxoplasma gondii infection.
NeuroImmunomodulation. 11:93-102.
GETZ, J. &
MONROY, F. (2005).
Effects of α-and
β-adrenergic
agonists on Toxoplasma gondii infection in murine
macrophages. Journal of Parasitology. 91:
193-195.
THOMPSON,
E., AVILES, H. & MONROY, F. P. (2008). Immuno characterization
of a
surface antigen of Toxoplasma
gondii.
Journal of Parasitology 94: 114-118.
Parasitology. 118 (2008) 134-138.
expression
during Toxoplasma gondii infection. Journal
of Parasitology. 94:
GOPAL, R., BIRDSELL, D. & MONROY, F.P. 2008. Regulation of
Toll-like receptors in intestinal epithelial cells by
stress and Toxoplasma gondii
infection. Parasite Immunology. 30: 563–576.