 |
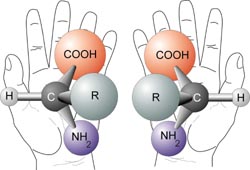
Enantiomers are mirror images of each other. That is, the same parts of the molecule are located in opposite positions, like the thumbs on a glove, so that they cannot be superimposed on each other but look the same in a mirror. For instance, hold up your left hand in front of a mirror and the image in the glass matches your right hand. Because of their opposite molecular structures, enantiomers polarize light in opposite directions, and are also called optical isomers.
Matching Shapes
In biological systems, the shape of a molecule is an essential characteristic when its function depends on matching the shape of other molecules, like a key in a lock. Such a match is particularly important for enzyme controled reactions, gene regulation, and cell communication. Even though most of the physical and chemical properties of optical isomers are identical, their shape is important when right- and left-handedness makes a difference: one optical isomer will facilitate a biochemical reaction and the other will not produce an effect or have a different effect.
Drugs
Many drugs depend for their intended beneficial action on a match between the shape of the medicinal molecule and some target molecule on the surface of a cell in the body. Albuterol is a bronchodilator used to aliviate the symptoms of asthma. One enantiomer of albuterol is effective, but the other is ineffective as a bronchodilator and counters the effect of the first. Ibuprofen is usually sold containing both enantiomers, but the active enantiomer relieves pain and reduces inflammation more rapidly than the racemic mixture.
Origin
Typical naturally occurring proteins, made of L amino acids, are known as left-handed proteins, whereas D amino acids produce right-handed proteins. Most scientists believe that Earth life's "choice" of left-handedness in amino acids was purely random, and that if carbon-based life forms exist elsewhere in the universe, their chemistry could theoretically have the opposite bias. However, some have suggested that early amino acids could have formed in comet dust. In this case, circularly polarized radiation (which makes up 17% of stellar radiation) could have caused the selective destruction of one form of amino acids, leading to a selection bias which ultimately resulted in all life on Earth using enantiomers of only one orientation.
Excerpted and adapted from: Brown, T. L., H. E. LeMay, Jr., and B. E. Bursten. 2006. Chemistry The Central Science. Pearson Education. 10th Edition. p 1038-1043, 1091-1092; Chirality (chemistry)