The role of complement in host defence has been established through genetic deficiencies of certain complement components, which may result in life-threatening recurrent bacterial infections or immune complex diseases. Such deficiencies shall be discussed later.
The role of complement in inflammation and tissue injury has become apparent through clinical investigations and discoveries that the pathogenesis of certain experimental inflammatory diseases is complement-dependent.
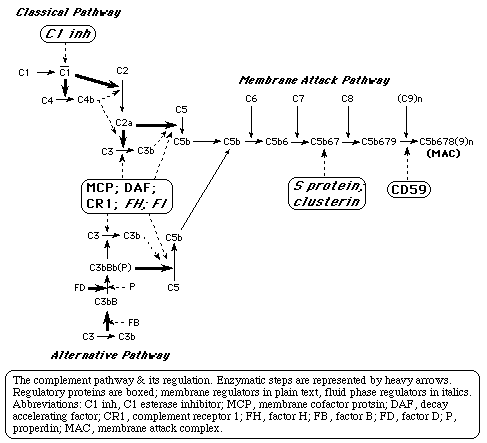
The complement system can be activated by two different pathways: the classical complement pathway and the alternative complement pathway.

The classical pathway is activated by the binding of antibody molecules (specifically IgM and IgG1, 2 and 3) to a foreign particle. This pathway is antibody-dependent.
The alternative pathway seems to be of major importance in host defence against bacterial infection because, unlike the classical pathway, it is activated by invading micro-organisms and does not require antibody. This pathway is antibody-independent.
The alternative pathway constitutes a humoral component of natural defence against infections, which can operate without antibodies. The six proteins C3, B, D, H, I, and P together perform the functions of initiation, recognition and activation of this pathway which results in the formation of activator-bound C3/C5 convertase.
The classical pathway functions to mediate the specific antibody response. It is as elaborately controlled as the alternative pathway, although it does lack the spontaneous initiation ability; i.e. the antibody independent recognition function, and the feedback amplification mechanism. Both activation pathways contain an initial enzyme that catalyses the formation of the target cell bound C3 convertase which in turn generates the C5 convertase. This results in the cleavage and activation of C5 and, therefore, in the assembly of the membrane attack complex (MAC). The MAC is assembled from five hydrophilic precursor proteins: C5, C6, C7, C8, and C9. Activation of the MAC is a consequence of the activity of either the classical or the alternative pathway on the surface of a cell. Through its metastable membrane binding site, the forming MAC binds firmly to target membranes owing to hydrophobic interactions with the lipid bilayer. The final events are the unfolding, the oligomerisation, or the polymerisation of C9, which causes the weakening of membrane structure, and the formation of transmembrane channels thus leading to osmotic lysis of the cell. MAC assembly is regulated by the S protein of plasma, and by homologous restriction factors of host cell membranes. Complement mediated lysis occurs in many kinds of cells: erythrocytes, platelets, bacteria, viruses possessing a lipoprotein envelope, and lymphocytes.
Complement activation occurs with each component sequentially. The sequence of component interactions of the complement system is dealt with in the following texts.
Click the Back button to return to the lesson.