
Why the body needs food
Your metabolism is the collection of chemical reactions that occur in your cells to sustain life. Some of these reactions use stored energy to build things up, which we call anabolism, while other reactions break things down, releasing energy that can be stored for future use, and this is called catabolism. Imagine that the hamburger you’re having for dinner, made of proteins, fats, and carbohydrates, is a collection of lego blocks of various colors and shapes. It took a lot of energy to organize those blocks into that complex structure, and breaking the blocks apart releases that energy and frees the blocks so that they can be built back up into new things. Your body does exactly that when you eat your food. Here's a brief video lecture that summarizes this concept.

Living things break down the three major categories of foods (proteins, fats, and carbohydrates) into their constituent parts, the individual lego blocks, for two reasons. 1) Once the food atoms and groups of atoms (molecules) are broken down, they can be built back up into the specific kinds of things the organism needs, like bone, muscle, skin, hair, feathers, fur, bark, leaves, etc. 2) Breaking down the food molecules releases the energy that was holding them together, and that released energy is temporarily stored by the cell for the re-building process. Each of these food types requires a different breakdown process, and we’ll look at those later, but the goal is the same–take the energy that held those food molecules together and release it so that it can be stored in a form that the cell can use later to build what it needs. The cell has a special kind of molecule for storing that energy, and it’s called ATP.
http://en.wikipedia.org/wiki/Adenosine_triphosphate

ATP (Adenosine tri-phosphate) is an important molecule found in all living things. Think of it as the “energy currency” of the cell. If a cell needs to spend energy to accomplish a task, the ATP molecule splits off one of its three phosphates, becoming ADP (Adenosine di-phosphate) + phosphate. The energy holding that phosphate molecule is now released and available to do work for the cell. When the cell has extra energy (gained from breaking down food that has been consumed or, in the case of plants, made via photosynthesis), it stores that energy by reattaching a free phosphate molecule to ADP, turning it back into ATP. The ATP molecule is just like a rechargeable battery. When it’s fully charged, it’s ATP. When it’s run down, it’s ADP. However, the battery doesn’t get thrown away when it’s run down–it just gets charged up again.
http://en.wikipedia.org/wiki/Adenosine_diphosphate
ATP ß à ADP + P + energy
Here’s what it looks like chemically. Each phosphate is a PO4 (oxygen has a charge of -2 and there are 4 of them, for a total of -8, and P has a charge of +5, so the net charge on the phosphate group is -3. If free H atoms, which are +1, get added to the O atoms that aren’t bonded to two things, then the net charge is zero.)
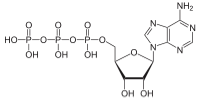
ß à
ATP ADP
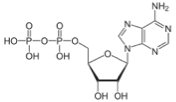
There are times when the cell needs even more energy, and it splits off another phosphate, so it goes from ADP, adenoside di-phosphate, to AMP, adenosine mono-phosphate.
ATP ß à ADP + P + energy ß à AMP + P + energy

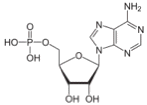
ß à
ADP AMP
There are other energy storage molecules in the cell, like NAD and FAD, but the ATP system is the most common, and the most important. Think of the others as different brands of rechargable batteries that do the same job. Next, we’ll explore some of the pathways that the body uses to break down foods of different types.
What about oxygen? Why do we need that? What happens if you put a glass over a candle? You starve the fire of oxygen, and the flame flickers out. If a metabolic reaction is aerobic, it requires oxygen. Buy why? Here's an analogy. Think about lighting a campfire. What do you need? You need fuel (the wood), you need heat (it's harder to light a fire when it's cold), and you need oxygen (because another word for burning is "oxidizing" and, as you might guess, it can only occur in the presence of oxygen). Oxidizing something causes it to lose electrons, which means that energy (the electrons) is released when you oxidize, or burn, a fuel. Your food is your fuel. You burn the fuel for energy. You need the oxygen to burn the fuel. This happens in the mitochondria.